Membrane electrolysis sodium hypochlorite jenereithara ke mochine o loketseng bakeng sa ho noa metsi disinfection, phekolo ea metsi a litšila, bohloeki le thibelo ea seoa, le tlhahiso ea indasteri, e hlahisoang ke Yantai Jietong Water Treatment Technology Co., Ltd., China Water Resources le Hydropower Research Institute, Univesithi ea Qingdao, Univesithi ea Yantai le litsi tse ling tsa lipatlisiso le liunivesithi. Ke mofuta oa mochini o hlahisang litharollo tse phahameng tsa sodium hypochlorite setšeng, o khotsofatsa haholo tlhoko ea lihlahisoa tse phahameng tsa sodium hypochlorite, mme o rarolla mathata a lipalangoang le polokelo. Jenereithara ea Membrane sodium hypochlorite e entsoeng ke Yantai Jietong Water Treatment Technology Co., Ltd. ke eona feela k'hamphani ea theknoloji Chaena e ka hlahisang lihlahisoa tse phahameng tsa sodium hypochlorite sebakeng sa marang-rang. Membrane electrolysis brine sodium hypochlorite jenereithara e ka hlahisa tharollo ea sodium hypochlorite e phahameng ea 4-12% ka loop e koetsoeng ea dosing le ho hlahisa ts'ebetso e ikemetseng ka botlalo.
Lintlha tse latelang ke Khopolo-taba ea Mosebetsi
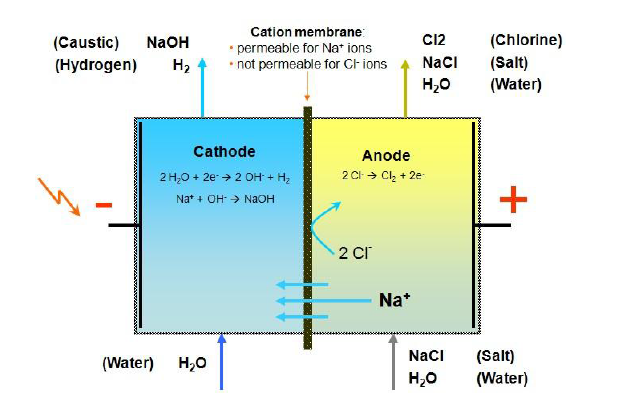
Molao-motheo oa electrolytic reaction ea membrane electrolysis cell ke ho fetolela matla a motlakase ho matla a lik'hemik'hale le electrolyze brine ho hlahisa NaOH, Cl2 le H2 joalokaha ho bontšitsoe setšoantšong se ka holimo. Ka kamoreng ea anode ea sele (ka lehlakoreng le letona la setšoantšo), brine e kenngoa ka har'a Na + le Cl- ka seleng, moo Na + e fallelang kamoreng ea cathode (lehlakoreng le letšehali la setšoantšo) ka lera la ionic le khethiloeng tlas'a ketso ea tefiso. Cl e tlase e hlahisa khase ea chlorine tlas'a anodic electrolysis. H2O ionization ka kamoreng ea cathode e fetoha H + le OH-, moo OH- e koetsoeng ke lera le khethiloeng la cation ka kamoreng ea cathode le Na + ho tloha kamoreng ea anode e kopantsoe ho etsa sehlahisoa sa NaOH, 'me H + e hlahisa hydrogen tlas'a cathodic electrolysis.


Nako ea poso: May-31-2024

