Jedium hypochlor
Jedium hypochlorte,
,
Tlhaloso
Gendrolysis Sodium ea Sodium Hypochlorte, Ltd. Gemrone Sodium Hypochlornite Genent Phekolo ea Tefo ea Yantai Jietong Thread

Molao-motheo oa ho Sebetsa
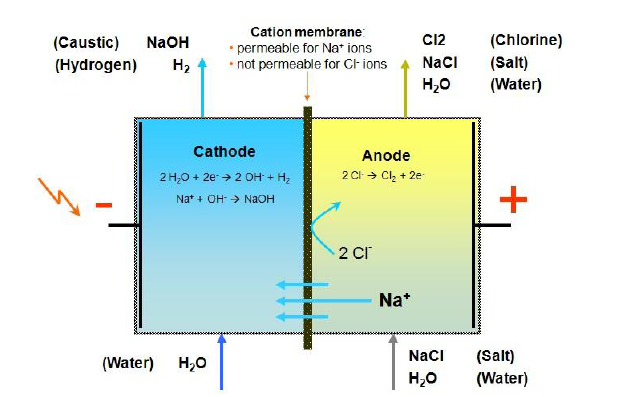
Molao-motheo oa mantlha oa karabelo ea motlakase oa motlakase ke ho fetola ea motlakase ka matla a lik'hemik'hale le motlakase ho tsoana setšoantšong se kaholimo. Ka kamoreng ea anode ea sele (ka lehlakoreng le letona la setšoantšo), boloi ba kenella ka kamoreng ea ho khetha li-iicre. Ntlha e ka tlase e hlahisa khase ea chlorine tlas'a Enodio Canodis. Ionization ea H2O Chamber e fetoha H + U Oh, Ah- Oh oh

Kopo
● Indasteri ea Chrerine-Alkali
● Ho arohana bakeng sa semela sa metsi
● Ho senyeha liaparo tse etsang semela
● Ho tlatlapa ho isa ho la hae le sebetsang ka lapeng, hotele, Sepetlele.
Lipere tsa Books
| Mohlala
| Chlorine (kg / h) | NACLO (kg / h) | Tšebeliso ea letsoai (kg / h) | DC Power Tšebeliso (Kw.) | Sebaka sa ho nka karolo (㎡) | Boima (Lithane) |
| JTWL-C1000 | 1 | 10 | 1.8 | 2.3 | 5 | 0.8 |
| JTWL-C5000 | 5 | 50 | 9 | 11.5 | 100 | 5 |
| JTWL-C10000 | 10 | 100 | 18 | 23 | 200 | 8 |
| JTWL-C15000 | 15 | 150 | 27 | 34.5 | 200 | 10 |
| JTWL-C20000 | 20 | 200 | 36 | 46 | 350 | 12 |
| JTWL-C30000 | 30 | 300 | 54 | 69 | 500 | 15 |
Nyeoe ea Morero
Jedium hypochlor
8tons / letsatsi la 10-12%

Jedium hypochlor
200kg / letsatsi 10-12%
 Kenyelletso
Kenyelletso
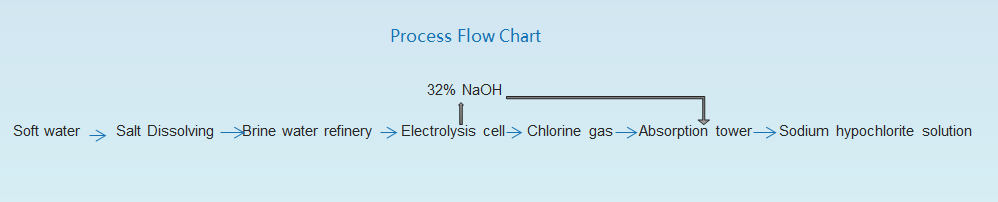
Gendrolysis Sodium ea Sodium Hypochlorte, Ltd. Ke mofuta oa mochini oa ho hlahisa tharollo ea sebaka sa sotium ea sodium ea sebaka sa sodium ea Sonpochlor hajoale Membrane Sodium Hypochlor Generantite Gecklonite By Phepelo ea Theknoloji Combotage International Sonpium Hypochlor sebakeng sa marang-rang. Membrane Electrolysis Brine Sodium Sodium Geneurment Thallo ea Sodium Hypochlorte le Loop e koetsoeng ea ho lahla le ho hlahisa ts'ebetso e ikemetseng ka botlalo.
Molao-motheo oa mantlha oa karabelo ea motlakase oa motlakase ke ho fetola ea motlakase ka matla a lik'hemik'hale le motlakase ho tsoana setšoantšong se kaholimo. Ka kamoreng ea anode ea sele (ka lehlakoreng le letona la setšoantšo), boloi ba kenella ka kamoreng ea ho khetha li-iicre. Ntlha e ka tlase e hlahisa khase ea chlorine tlas'a Enodio Canodis. Ionization ea H2O Chamber e fetoha H + U Oh, Ah- Oh oh









